Potential COVID-19 Vaccine is in Development
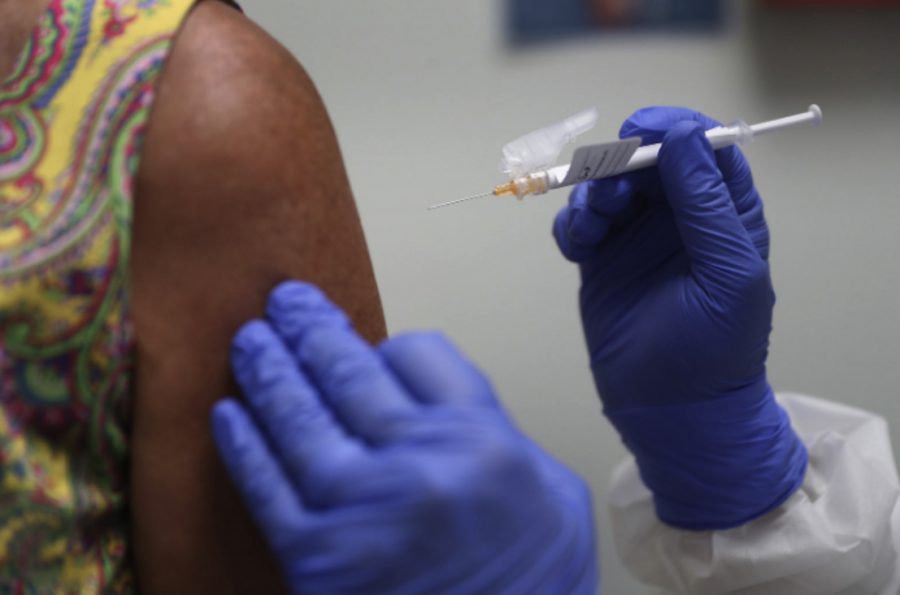
A Swedish Doctor injects the Coronavirus vaccine into an AstraZeneca volunteer. The many stages of vaccine development depend on the success of previous trials.
AstraZeneca, a Swedish pharmaceutical company, started the third and last phase of the Coronavirus (COVID-19) vaccine clinical trial on Sept. 1. The vaccine would be administered world wide if all trials go well. On Sept. 9, however, the vaccine resulted in one of the volunteers developing an unknown illness, causing a pause in the trials according to CNN.
The vaccine could potentially end the pandemic and make schools and other public areas a safe environment once again, according to CNN.
While it is unsure when the vaccine can be administered to the public, AstraZeneca and eight other companies signed a pledge to ensure that they would not be influenced by any governments to prematurely release the vaccine. This means the vaccine will continue to be developed under the promise of ethicality, but be administered as a regular flu shot would, even 1if it means postponing the release, according to ABC 7.
“We, the undersigned biopharmaceutical companies, want to make clear our on-going commitment to developing and testing potential vaccines for COVID-19 in accordance with high ethical standards and sound scientific principles,” the pledge reads, which was signed by the CEOs of AstraZeneca, BioNTech, GlaxoSmithKline, Johnson & Johnson, Merck, Moderna, Novavax, Pfizer and Sanofi.
In order for the pandemic to end, 70% of the population would have to be immune, whether it is from already being exposed or vaccination. This is equivalent to about 200 million Americans and 5.6 billion people worldwide, according to the New England Journal of Medicine.
“I have a lot of faith that the FDA technical people, who sit through one government after another doing their job, are going to do their job,” Harvard research professor of public health Dr. Barry Bloom said in an interview with the New England Journal of Medicine.
Federal health agencies are expecting the vaccine to be gradually introduced to the public in January and then slowly become available to any American who wants the vaccine, according to The Orange County Register.
Your donation will support the student journalists of Portola High School. Your contribution will allow us to purchase equipment and cover our annual website hosting costs.

Sidra Asif is the Back Page Editor and Social Media Manager for her third year on the Pilot. Outside of creating content for the newspaper, you can find...