Clinical Trials for COVID-19 Vaccine Underway for Children 12 and Older
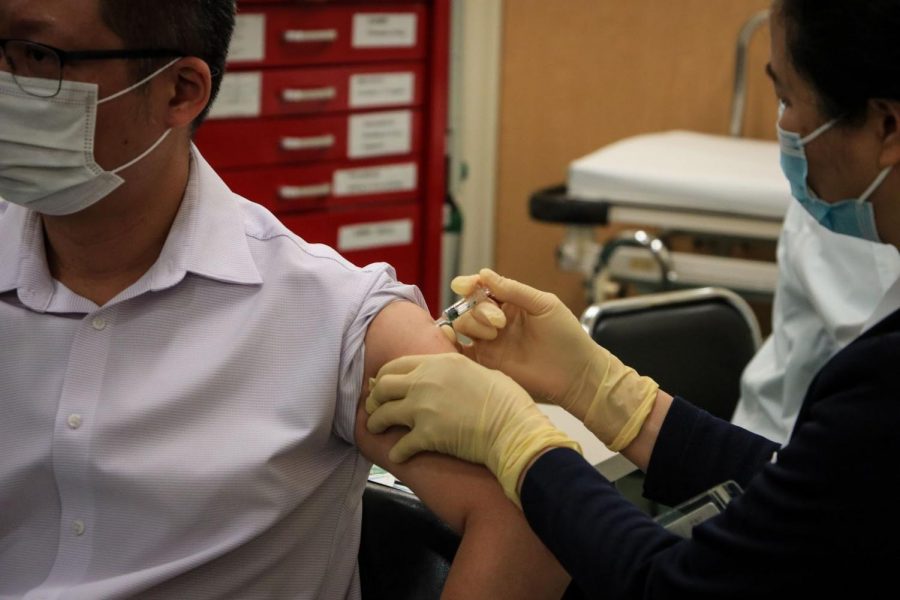
Photo Courtesy of Macau Photo Agency on Unsplash
Phase 2, the final scheduled phase of vaccine rollout, includes all people 16 or older not already recommended for vaccination in Phases 1a, 1b or 1c, according to the CDC. The Advisory Committee on Immunization Practices is “closely monitoring clinical trials in children and adolescents and will consider recommendations for use when a COVID-19 vaccine is authorized for use in persons aged <16 years,” according to the CDC website.
As of March 8, Moderna and Pfizer-BioNTech are in the midst of conducting clinical trials of the COVID-19 vaccine for children 12 to 17 and 12 to 15, respectively, with both companies expecting results “by the summer,” according to the New York Times.
“We really want to make sure they’re safe and well-tolerated in children, particularly when there’s a low risk-benefit ratio,” Richard Malley, a senior physician in the Division of Infectious Diseases at Boston Children’s Hospital, said in an interview with the Washington Post.
According to Moderna’s participant recruitment page for the clinical trials, the trials are 13 months long. Volunteers received two injections in the upper arm one month apart and will receive calls from a study doctor monthly and after each injection.
Moderna also asks its study volunteers to keep an eDiary app on their smartphones to complete weekly entries about any COVID-19 symptoms for the duration of the study, according to the same recruitment page.
Children are not expected to receive the vaccine until at least late summer, according to the New York Times.
“I think that having this specific timeline is not really great because it prevents under-16-year-olds from getting the vaccine, but I do think it’s still a good idea to have more at-risk people to get the vaccine faster,” freshman Elise Ngo said. “I feel like I missed a lot with my freshman year, and it’s a little sad, but it’s just the way things are, and I just have to hope things go back to normal soon.”
Despite the lack of vaccination among children, the CDC’s most recent guidelines urge that K-12 schools reopen except in cases of the highest transmission levels, which are defined as either having 10% of COVID-19 tests come back positive over a seven-day period or when there are 100 cases per 100,000 people in the community over seven days.
As of March 4, Orange County was at seven cases per 100,000 people, according to NBC Los Angeles.
“The risk is to the adults, and teachers will have had their vaccines, the staff will have had their vaccines and hopefully all the people who are vulnerable in their homes will have had vaccines,” New York Times science reporter Apoorva Mandavilli said in “The Daily” podcast.
Your donation will support the student journalists of Portola High School. Your contribution will allow us to purchase equipment and cover our annual website hosting costs.

Kate Hayashi is the co-editor-in-chief of the Portola Pilot. She draws all her writing inspiration from Michael Barbaro's "hmms" in “The Daily.” Outside...